This blog is cross-posted from the Department of Chemical Engineering and Biotechnology website.
One area of our research that is perhaps more obviously linked to a viral pandemic is the field of disease diagnostics. Several of our biotechnology-focused groups already work on developing accurate testing for a variety of diseases, with our Analytical Biotechnology group and Open Bioeconomy Lab focusing specifically on testing for so-called tropical diseases, such as dengue and malaria.
The groups, led by our outgoing Head of Department, Professor Lisa Hall CBE, and Shuttleworth Fellow Dr Jenny Molloy, are dedicated to building diagnostic capabilities in countries that are most affected by such diseases. Rather than simply develop tests here that could be sold to healthcare professionals abroad, their focus is on supplying and supporting the basic infrastructure needed for the tests to be produced by clinics within the countries themselves.
To contain the spread of a new virus, access to fast, affordable testing on a vast scale is essential, and as COVID-19 began to spread across the world, the researchers quickly shifted their focus to developing testing capacity for this new coronavirus.
The concept of local manufacturing has several advantages over the existing traditional diagnostic supply chain, which requires importation of tests or test reagents at overseas prices that are often prohibitively expensive in comparison to local purchasing power.
Producing the tests in-country can significantly lower costs could be substantially lowered, making them more affordable for use at the scale needed to tackle COVID-19.
Local manufacturing also builds resilience and autonomy into a supply chain that is challenging and prone to disruption even at the best of times. Many researchers in Africa, Asia and Latin America routinely face long waits for the reagent kits and enzymes they need – from weeks to months – in addition to disruption of the cold chain for reagents delivered on ice, and challenges with customs, importation paperwork and public or institutional procurement systems. That problem is exacerbated during the pandemic when demand is high; normal supply chains are struggling to cope with demand and many countries that manufacture reagents are imposing export controls.
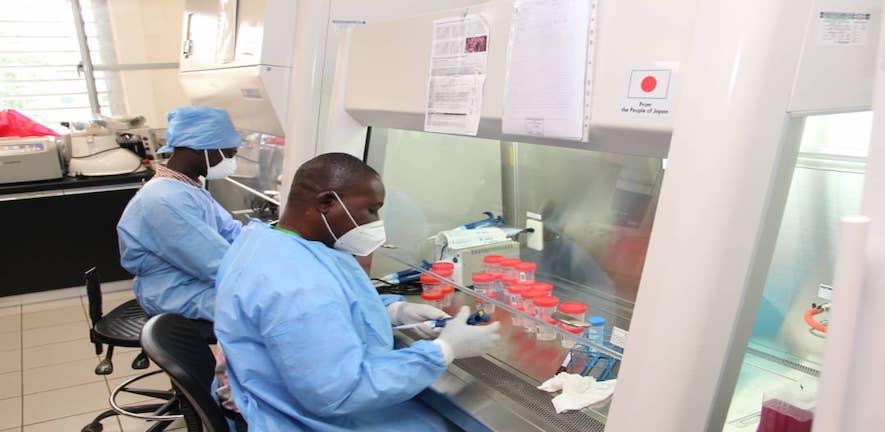
Hall and Molloy are working with collaborators at the Technical University of Denmark (DTU) and three Ghanaian research institutions, including two national COVID-19 testing centres, to begin clinical assessment of a rapid, point-of-care test for COVID-19 RNA developed by DTU. By working with local laboratories in Ghana, the Cambridge researchers plan to develop the capacity there for manufacturing of the enzymes used in the test that has been developed by the Danish University.
The project builds on their existing work to develop low-cost protocols for enzyme manufacturing. They have developed a system for producing and purifying enzymes for nucleic acid tests by attaching the enzymes needed for the assay onto silica particles. The silica can be cheaply obtained from beach sand or other locally available material.
The enzymes they make are tagged with a bright-pink coloured protein that offers visual reassurance across all steps of the production and purification process, showing that the enzyme has been produced correctly. The whole process has been designed to allow distributed local production without high capital investment, offering convenience, ease of production and quality control with substantial production cost savings.
Researchers in the Analytical Biotechnology group, including PhD student Dushanth Seevaratnam, and Research Associate Dr Cassi Henderson, have already undertaken clinical trials in Ghana and Malaysia, using these enzymes in nucleic acid testing to detect species of malaria, and the tests are adaptable to many infectious diseases.
“The basic test components remain the same but the sequence for different RNA or DNA is targeted for whatever disease you are looking to test for, be that Ebola, malaria, or the SARS-CoV-2 virus that causes COVID-19,” says Professor Hall.
“Infectious diseases remain a major cause of morbidity and mortality in low-income countries. As well as COVID-19, these include food and waterborne diseases (bacterial diarrhoea, enteric fever, hepatitis A), vector-borne diseases (malaria, dengue, chikungunya and Zika), zoonotic diseases (leptospirosis, rabies) respiratory infections (influenza-like illnesses, pneumonia, tuberculosis) and HIV infection.
There is a lack of diagnostic tests to distinguish febrile disease like COVID-19, and with patients presenting fever and a wide range of nonspecific symptoms, which are difficult to diagnose without specialist laboratory tests, doctors and medical practitioners are having to diagnose on the basis of probability, resulting in empirical treatment, which may be unnecessary, incorrect or potentially even harmful.”
The collaboration to develop affordable diagnostics for the entire COVID-19 care pathway also includes Dr Tony Jackson from the Department of Biochemistry at the University of Cambridge, who is looking at a novel single chain antibody for the team to develop an immunoassay. This will allow them to design a test which indicates if people have previously had COVID-19.
The antibody technology can be adapted for local distributed production in the same way that the polymerase enzyme is produced for the nucleic acid test, which could be a game-changer in terms of rapid access for the next pandemic.
Dr Molloy has been working with labs in Ghana, Cameroon and Ethiopia for two years to build capacity for enzyme manufacturing; leveraging a collection of 16 off-patent enzymes, which her group are currently synthesising in collaboration with US company Ginkgo Bioworks and Stanford University as “ready-to-use”, plus over 100 useful blocks of DNA to build new functions. These will be shipped to a network of labs for research, including those conducting research in diagnostics who will share back the results of their experiments to enable the whole network to move forward quickly to optimised protocols.
“What is exciting about the Reclone network is the speed at which partnerships have developed between academia, government and the private sector in both Latin America and Africa,” says Dr Molloy. “The scientists leading these initiatives have a sense of urgency but are cognisant of the lengthy timelines involved in getting manufacturing efforts off the ground in comparison to the speed of the pandemic. They are committed to a long-term vision for building biotechnology capacity and increasing pandemic preparedness that could have far-reaching impact in their countries and regions”.
Professor Hall, whose past work has included the research behind widely used glucose test strips for diabetes, has recently been focusing on breaking the barrier to local manufacturing of diagnostics in low- and middle-income countries, working with Ghana, Malaysia and the Philippines.
Her and Dr Molloy’s next research steps are now looking at advancing the manufacture of a regulator-approved diagnostic device in countries currently without all the enabling infrastructure. “We are looking for an affordable device at a cost that is equivalent to a bowl of rice (or local equivalent), which would help build local economies and education without needing to rely on foreign aid,” explains Hall.
This goal requires research and innovation in financing models and partnerships as well as a roadmaps and guidance for manufacturers and regulators to ensure high-quality products meet international guidelines. There are numerous other challenges to overcome in how those tests are then applied in the health system. Dr Molloy is working with the Ethiopian Biotechnology Institute to scale up COVID-19 testing using open source robotics hardware, as well as consideration of economic, ethical, legal and social dimensions at a systems level.